What is the EGFR and KRAS, and what is their importance in the progression of colorectal cancer?
- EGFR (Epidermal Growth Factor Receptor), also synonymously ERBB (ERythroBlastic leukemia); ERBB1; HER1 (Human epidermal growth factor Receptor), is a transmembrane receptor whose activation by ligand binding triggers the intracellular signaling cascade resulting in the impact on the gene expression, which leads, simply speaking, to the cell proliferation. Eventual dysregulation of this pathway may thus lead to tumor progression.
- KRAS, synonymously also K-RAS or K-ras, gene product KRAS (Kirsten RAt Sarcoma viral oncogene homolog), is a protein with GTPase activity, which is part of the EGFR signaling cascade. Therefore, it participates on signal transmission from the activated receptor to the nucleus. Activating mutations of this gene thus lead to stimulation of the mentioned signaling pathway, independently of the status of the EGFR. This mutation occurs in about 35% of colorectal cancers.
How important is the examination of expression of EGFR immunohistochemistry and molecular genetic testing of KRAS in colorectal cancer?
After determining the role of EGFR signaling pathways in the progression of colorectal cancer, monoclonal antibodies against this receptor were developed for the treatment (anti-EGFR MoAb), that should result in inhibition of the signaling cascade by blocking this receptor, and thus lead to the inhibition of tumor cell growth. The mentioned examination should enable the selection of patients for therapy using these antibodies.
- Thanks to clinical trials, the meaning of immunohistochemical expression of EGFR, despite initial enthusiasm, has proved almost zero. Positive proof is no guarantee of an advantageous response to treatment, as well as EGFR negativity does not exclude a positive therapeutic response to treatment using anti-EGFR moAb.
- On the contrary, the presence of activating mutations of KRAS gene has been shown in clinical studies as an important determinant of tumor resistance to the treatment by anti-EGFR moAb. The reason is, that in this case, the abnormal KRAS protein still stimulates the cell to proliferation, in spite of the inhibition of EGFR, thanks to the change of its own structure, the KRAS protein is disengaged from a regulatory influence of EGFR. This means, that only patients with tumors whose cells contain mutated KRAS gene only, can expect therapeutic success.
-
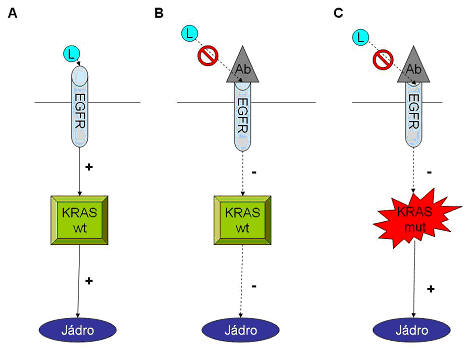 EGFR KRAS scheme
EGFR KRAS scheme- in the normal state, EGFR is activated by the binding of ligand, leading to stimulation of intracellular signaling pathway, which includes KRAS, which leads to cell proliferation.
- antibody against the EGFR prevents its activation, which prevents tumor cell proliferation in the non-mutated KRAS (wt) gene.
- activating mutation of KRAS gene results in stimulation of cells to proliferation in the absence of activation of EGFR.
What methods does our laboratory use to detect KRAS mutations?
Examination of KRAS gene mutations can be performed on fresh, frozen, formalin-fixed and paraffin-embedded tissue. For each sample, we perform quality control of DNA using an amplification of control genes.
At present, we analyze mutations in exon 1 and adjacent exon-intron joints of the KRAS gene by PCR amplification and direct sequencing. Especially, we focus on identification of twelve activating mutations in codons 12 and 13.
We also provide detection of the seven most common KRAS gene mutations using the certified kit TheraScreen KRAS Mutation Kit (DxS Diagnostic/QIAgen). This examination combines technological principles ARMS and Scorpions and detect mutations under conditions of real-time PCR. The main advantage of this examination method is its high sensitivity. It can detect a mutated clone which represents only 1% on the background of the non-mutated DNA.
What is the best way to order a molecular genetic analysis of KRAS?
The molecular genetic testing requires sending a paraffin block with a tumor, preferably to the following address:
Prof. Ondřej Daum, MD, PhD.
Bioptická laboratoř s.r.o.
Mikulášské nám. 4
326 00 Pilsen
In case you are our client, and material from colorectal cancer was examined in the Bioptická laboratoř s.r.o. (and respective blocks are archived here), you can simply send a written request for an examination to the above mentioned address or by e-mail to the following address: daum@medima.cz
In order to ensure optimal examination process and to shorten the time as much as possible, we ask for this data:
- the explicit indication of application for molecular genetic analysis of KRAS
- specification of particular antibody, using which the patient should be treated (e.g. cetuximab, panitumumab)
Reference
- Daum M, Šíma R, Němcová J, Beneš Z, Michal M. Současné možnosti predikce odpovědi na biologickou terapii u kolorektálního karcinomu. Čes a Slov Gastroent a Hepatol 2009; 63(1): 25-28.
- Amado RG, Wolf M, Peeters M, Van Cutsem E, Siena S, Freeman DJ, Juan T, Sikorski R, Suggs S, Radinsky R, Patterson SD, Chang DD. Wild-type KRAS is required for panitumumab efficacy in patients with metastatic colorectal cancer. J Clin Oncol. 2008 Apr 1;26(10):1626-34.
- Baselga J, Rosen N. Determinants of RASistance to anti-epidermal growth factor receptor agents. J Clin Oncol. 2008 Apr 1;26(10):1582-4.
- Chung KY, Shia J, Kemeny NE, Shah M, Schwartz GK, Tse A, Hamilton A, Pan D, Schrag D, Schwartz L, Klimstra DS, Fridman D, Kelsen DP, Saltz LB. Cetuximab shows activity in colorectal cancer patients with tumors that do not express the epidermal growth factor receptor by immunohistochemistry. J Clin Oncol. 2005 Mar 20;23(9):1803-10.
- Cunningham D, Humblet Y, Siena S, Khayat D, Bleiberg H, Santoro A, Bets D, Mueser M, Harstrick A, Verslype C, Chau I, Van Cutsem E. Cetuximab monotherapy and cetuximab plus irinotecan in irinotecan-refractory metastatic colorectal cancer. N Engl J Med. 2004 Jul 22;351(4):337-45.
- De Roock W, Piessevaux H, De Schutter J, Janssens M, De Hertogh G, Personeni N, Biesmans B, Van Laethem JL, Peeters M, Humblet Y, Van Cutsem E, Tejpar S. KRAS wild-type state predicts survival and is associated to early radiological response in metastatic colorectal cancer treated with cetuximab. Ann Oncol. 2008 Mar;19(3):508-15.
- Hamilton SR. Targeted therapy of cancer: new roles for pathologists in colorectal cancer. Mod Pathol. 2008 May;21 Suppl 2:S23-30.
- Lievre A, Bachet JB, Le Corre D, Boige V, Landi B, Emile JF, Côté JF, Tomasic G, Penna C, Ducreux M, Rougier P, Penault-Llorca F, Laurent-Puig P. KRAS mutation status is predictive of response to cetuximab therapy in colorectal cancer. Cancer Res. 2006 Apr 15;66(8):3992-5.
- Saltz L. Epidermal growth factor receptor-negative colorectal cancer: is there truly such an entity? Clin Colorectal Cancer. 2005 Nov;5 Suppl 2:S98-100.
- Saltz LB, Meropol NJ, Loehrer PJ Sr, Needle MN, Kopit J, Mayer RJ. Phase II trial of cetuximab in patients with refractory colorectal cancer that expresses the epidermal growth factor receptor. J Clin Oncol. 2004 Apr 1;22(7):1201-8.
- Scartozzi M, Bearzi I, Berardi R, Mandolesi A, Fabris G, Cascinu S. Epidermal growth factor receptor (EGFR) status in primary colorectal tumors does not correlate with EGFR expression in related metastatic sites: implications for treatment with EGFR-targeted monoclonal antibodies. J Clin Oncol. 2004 Dec 1;22(23):4772-8.
- Spano JP, Milano G, Vignot S, Khayat D. Potential predictive markers of response to EGFR-targeted therapies in colorectal cancer. Crit Rev Oncol Hematol. 2008 Apr;66(1):21-30.
