Hemochromatosis type I is a disorder with autosomal recessive inheritance which manifests by increased deposition of iron in different organs, but mainly in the liver. It has been known for more than 100 years and until recently was considered to be a rare disease. Only the development of molecular methods in recent years has revealed that it is one of the most common genetic disorders of all.
Hemochromatosis is associated with point mutations in the gene called HFE which is localized on the chromosome 6 (6p21.3). This gene is localized on the chromosome 6 in the region p21.3 and encodes a protein which inhibits the activity of the transferrin receptor and thereby prevents excessive accumulation of iron in cells.
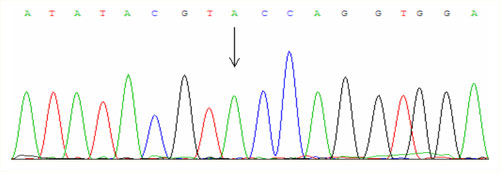
The most important point mutation is called C282Y and causes a substitution of cystein for tyrosine at the position 282. This mutation in the European Caucasian population occurs in the heterozygous state approximately in 10 % of people and one in four hundred inhabitans is homozygous C282Y. The disease manifests only in the homozygous state and approximately 87 % of patients with hemochromatosis is homozygous precisely in this mutation.
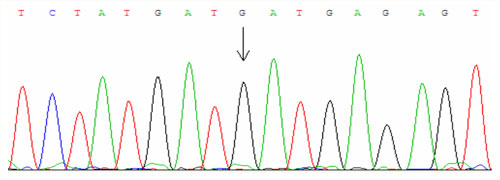
The second essential mutation is called H63D and causes the substitution of histidine for aspartic acid at position 63. About 25 % of the European population is heterozygous for this mutation and one in 130 inhabitants is homozygous. The disease manifests in the homozygous state or in trans-combination with C282Y.
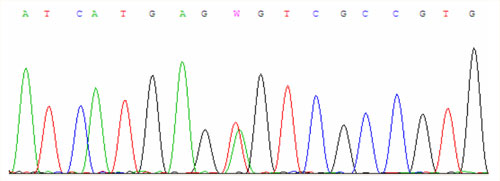
Another mutation is called S65C when there is a substitution of serine for cysteine at the position 65. In the European Caucasian population it occurs in the heterozygous state approximately in 1.5 % of people. The disease manifests in the homozygous state or in trans-combination with previous mutations.
There are more point mutations that occur in the gene HFE, which, however, appears to have no significant influence on the protein function.
Examination
By the means of molecular biology, it is possible to detect point mutations mainly by PCR in combination with restriction analysis, sequencing or by analysis of denaturating curves.
For examinations we use PCR amplification and direct sequencing (fig. 1, fig. 2, fig. 3).
References
- Meadows CA, Phillips M, Huang MY, Lyon E. Simultaneous detection of C282Y and H63D hemochromatosis mutations using LCRed 640 and LCRed 705 labeled hybridization probes. In: Rapid cycle real-time PCR, methods and applications. Meuer S, Wittwer C, Nakagawara K (Eds.). Springer Verlag Germany 2001;127-134.
- Hanson EH, Imperatore G, Burke W. HFE gene and hereditary hemochromatosis: a HuGE review. Human Genome Epidemiology. Am J Epidemiol. 2001;154(3):193-206.
- Zdarsky E, Horak J, Stritesky J, Heirler F. Hemochromatosis. Determination of the C282Y mutation frequency in the population of the Czech Republic and sensitivity of hemochromatosis detection using Guthrie cards. Cas Lek Cesk. 1999;138(16):497-499.
- Kankova K, Jansen EH, Marova I, Stejskalova A, Pacal L, Muzik J, Vacha J. Relations among serum ferritin, C282Y and H63D mutations in the HFE gene and type 2 diabetes mellitus in the Czech population. Exp Clin Endocrinol Diabetes. 2002;110(5):223-229.
- Cimburova M, Putova I, Provaznikova H, Horak J. Hereditary hemochromatosis: detection of C282Y and H63D mutations in HFE gene by means of guthrie cards in population of Czech Republic. Genet Epidemiol. 2002;23(3):260-263.