The c-kit gene localized on the chromosome 4 in the region q11-12 encodes a transmembrane receptor tyrosine kinase, homologous to PDGFR (platelet derivated growth factor receptor), which is activated by dimerization and autophosphorylation after a binding of SCF (stem cell factor) ligand SCF. The product of c-kit gene plays a significant role in the development of mastocytes, melanocytes, stem cells, red and white blood cells, germ cells and cells of Cajal.
Mutations which cause ligand-independent activaton were found in a range of neoplastic processes, such as mastocytosis, myeloproliferative diseases, acute myelogenous leukemia, sinonasal lymphoma and gastrointestinal stromal tumors (GIST). The spectrum of mutations is relatively wide. It includes particularly point mutations, in-frame deletions and insertions and covers a number of exons of the c-kit gene. In principle, it is possible to divide them into the mutations affecting a regulatory function and mutations affecting enzymatic part of a protein.
The mutation of the c-kit gene is found in 30 - 50 % of patients with GIST (in our laboratory ca. 40 %). It concerns mostly the mutations in exon 11 (ca. 70 %) which encodes a sub-membrane domain with a regulatory function and in exon 9 (ca. 15 %) encoding an extracellular domain. The c-kit gene mutations occur rarely also in exons 13 and 17 which encode a domain with tyrosin kinase activity.
The receptor tyrosin kinases may be the target for a targeted therapy by their specific inhibitors. One of them is imatinib mesylate (STI 571, Glivec) originally developed for ABL inhibition (abelson murine leukemia viral oncogene homolog 1) of tyrosin kynase of the fusion gene BCR-ABL in patients with a chronic myelogenous leukemia which, however, inhibits selectively also PDGFR and KIT tyrosin kinases. A significant regresion or stabilization of tumors have been observed in early studies of patients with GIST, in which this type of therapy have been used. Recent tests also indicate a correlation between the response to imatinib mesylate treatment and mutated domain or a concrete exon. The results so far indicte that the patients with mutated regulatory protein domain, mostly with the mutation in exon 11, have a better response to treatment than the patients with mutations in enzymatic domain the KIT protein.
Examination
In our laboratory, we performe an examination of mutations in exons 9, 11, 13 and 17 using PCR and direct sequencing.
-
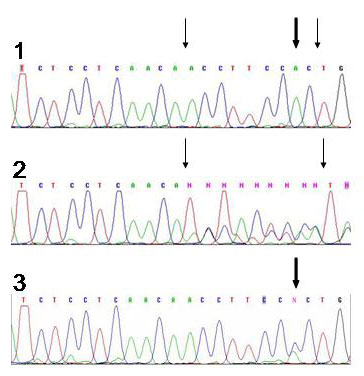 Fig.1
Fig.1Sequencing analysis of mutations in exon 11 using capillary sequencer ABI Prism 3100 Avant.
1) Part of the sequence without mutation
2) Part of the sequence with a deletion mutation of 9 bases
3) Part of the sequence with a point mutation
Thick arrows show the placement of point mutation, thin arrows the placement of deletion.
Analytical sensitivity and specificity of the sequencing: 99%.
Limitations:
In the case of the analysis of somatic mutations by sequencing the mutations will not be detected, if the altered cell line is not represented by at least 20%.
References
- Hirota S, Isozaki K, Moriyama Y, Hashimoto K, Nishida T, Ishiguro S, Kawano K, Hanada M, Kurata A, Takeda M, Muhammad Tunio G, Matsuzawa Y, Kanakura Y, Shinomura Y, Kitamura Y. Gain-of-function mutations of c-kit in human gastrointestinal stromal tumors. Science. 1998;279(5350):577-80.
- Miettinen M, Lasota J. Gastrointestinal stromal tumors--definition, clinical, histological, immunohistochemical, and molecular genetic features and differential diagnosis. Virchows Arch. 2001;438(1):1-12.
- Joensuu H, Roberts PJ, Sarlomo-Rikala M, Andersson LC, Tervahartiala P, Tuveson D, Silberman S, Capdeville R, Dimitrijevic S, Druker B, Demetri GD. Effect of the tyrosine kinase inhibitor STI571 in a patient with a metastatic gastrointestinal stromal tumor. N Engl J Med. 2001;344(14):1052-6.
- Heinrich MC, Rubin BP, Longley BJ, Fletcher JA. Biology and genetic aspects of gastrointestinal stromal tumors: KIT activation and cytogenetic alterations. Hum Pathol. 2002;33(5):484-95.
- Wardelmann E, Neidt I, Bierhoff E, Speidel N, Manegold C, Fischer HP, Pfeifer U, Pietsch T. c-kit mutations in gastrointestinal stromal tumors occur preferentially in the spindle rather than in the epithelioid cell variant. Mod Pathol. 2002;15(2):125-36.
- Heinrich MC, Corless CL, Demetri GD, Blanke CD, von Mehren M, Joensuu H, McGreevey LS, Chen CJ, Van den Abbeele AD, Druker BJ, Kiese B, Eisenberg B, Roberts PJ, Singer S, Fletcher CD, Silberman S, Dimitrijevic S, Fletcher JA. Kinase mutations and imatinib response in patients with metastatic gastrointestinal stromal tumor. J Clin Oncol. 2003;21(23):4342-9.
- Bauer S, Corless CL, Heinrich MC, Dirsch O, Antoch G, Kanja J, Seeber S, Schutte J. Response to imatinib mesylate of a gastrointestinal stromal tumor with very low expression of KIT. Cancer Chemother Pharmacol. 2003;51(3):261-5.
- Lasota J, Kopczynski J, Sarlomo-Rikala M, Schneider-Stock R, Stachura T, Kordek R, Michal M, Boltze C, Roessner A, Stachura J, Miettinen M. KIT 1530ins6 mutation defines a subset of predominantly malignant gastrointestinal stromal tumors of intestinal origin. Hum Pathol. 2003;34(12):1306-12.
