Renal tumors include about 3% of all malignant neoplasia of adulthood. The overwhelming majority of these tumors fall into renal carcinoma (RC). Renal epithelial tumors can be, according to Heidelberg and UICC97 classification, divided into several types. It is a conventional RC, papillary RC, chromophobe RC, RC from the collecting ducts and unclassified type of RC. Benign epithelial tumors are then papillary renal adenoma, renal oncocytoma and metanephric adenoma and related lesions. The histological type of RC is considered to be an important prognostic factor. The distinction between different RC types only via histology finding, however, is problematic in some cases. For these reasons, in order to perform an exact classification of tumors and to determine the prognosis, it is appropriate to obtain further information, e.g. analysis of tumors at the molecular level.
Works based on molecular genetic and cytogenetic data, constitute the classification of each RC type according to changes in the number of individual parts or whole chromosomes. It was found that various histological types include specific losses or gains of some chromosomes. For example, the main marker of the conventional RC is the loss of the short (p) arm of chromosome 3, eventually the gain of chromosome 5q along with the deletion of some of chromosomes 6q, 8p. 9p, 14q, papillary RC in a high percentage of cases obtain at least two of chromosomes 3q, 7, 8, 12, 16, 17 and 20, and chromophobe RCs show the loss of chromosomes 1, 2, 6, 10, 13, and 17.
Probably the most appropriate methodology to obtain comprehensive information on the genetic profile of the sample, is the CGH and its variants. However, this method is relatively expensive. It has specific requirements on the quality of material and, requires special equipment. However, similar information can be obtained using other methods, such as microsatellite analysis (loss of heterozygosity analysis - loss of heterozygosity - LOH) or fluorescence in-situ hybridization (FISH).
Examination
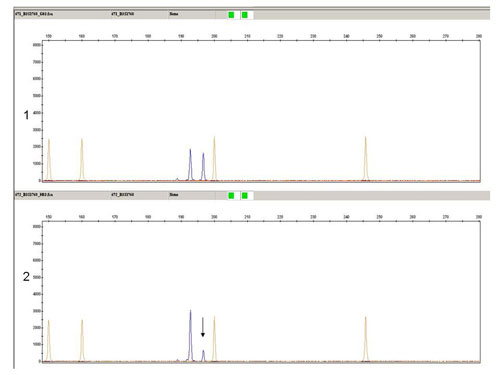
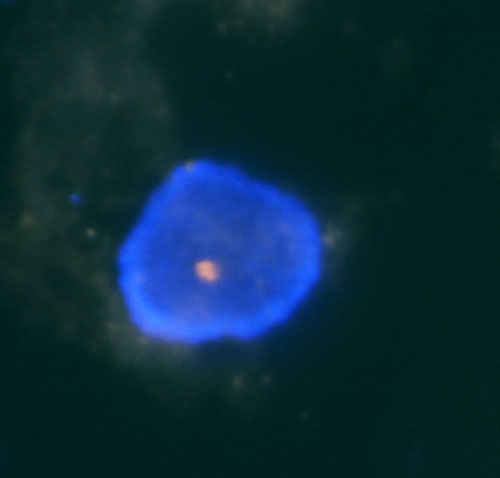
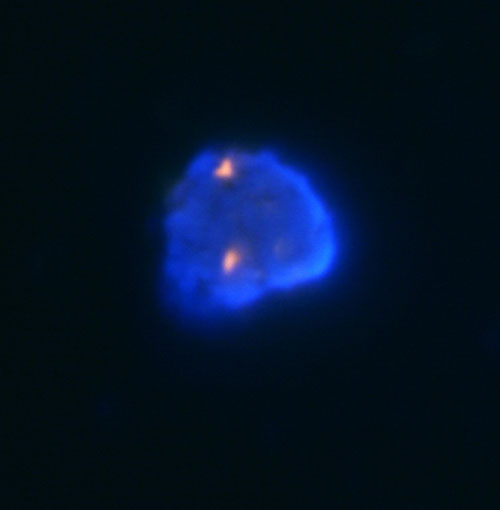
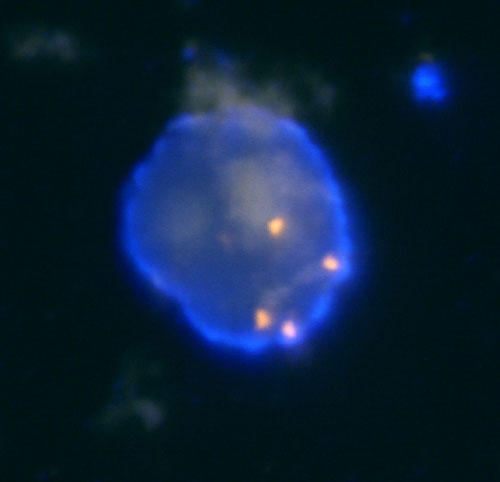
In our laboratory, we perform the detection of losses and gains of genetic material through the analysis of LOH and FISH. For the analysis of LOH when investigating the state of chromosomes 1 and 3, we use microsatellite markers D1S1656 (1q), D3S1300 (3p) and D3S1768 (3p). Fragmentation analysis of the PCR products is carried out on an ABI PRISM 3130xl genetic analyzer and is evaluated by the GeneMapper program (Fig. 1). Then, using the FISH, using centromeric probes and, using locus specific probes of chromosomes 13 and 21, we investigate the state of chromosomes 1, 2, 6, 7, 10, 17 (Fig. 2a, 2b and 2c).
References
- Speicher MR, Schoell B, du Manoir S, Schrock E, Ried T, Cremer T, Storkel S, Kovacs A, Kovacs G. Specific loss of chromosomes 1, 2, 6, 10, 13, 17, and 21 in chromophobe renal cell carcinomas revealed by comparative genomic hybridization. Am J Pathol. 1994;145(2):356-64.
- Bugert P, Kovacs G. Molecular differential diagnosis of renal cell carcinomas by microsatellite analysis. Am J Pathol. 1996;149(6):2081-8.
- Corless CL, Aburatani H, Fletcher JA, Housman DE, Amin MB, Weinberg DS. Papillary renal cell carcinoma: quantitation of chromosomes 7 and 17 by FISH, analysis of chromosome 3p for LOH, and DNA ploidy. Diagn Mol Pathol. 1996;5(1):53-64.
- Kattar MM, Grignon DJ, Wallis T, Haas GP, Sakr WA, Pontes JE, Visscher DW. Clinicopathologic and interphase cytogenetic analysis of papillary (chromophilic) renal cell carcinoma. Mod Pathol. 1997;10(11):1143-50.
- Kovacs G, Akhtar M, Beckwith BJ, Bugert P, Cooper CS, Delahunt B, Eble JN, Fleming S, Ljungberg B, Medeiros LJ, Moch H, Reuter VE, Ritz E, Roos G, Schmidt D, Srigley JR, Storkel S, van den Berg E, Zbar B. The Heidelberg classification of renal cell tumours. J Pathol. 1997;183(2):131-3.
- Storkel S, Eble JN, Adlakha K, Amin M, Blute ML, Bostwick DG, Darson M, Delahunt B, Iczkowski K. Classification of renal cell carcinoma: Workgroup No. 1. Union Internationale Contre le Cancer (UICC) and the American Joint Committee on Cancer (AJCC). Cancer. 1997;80(5):987-9.
- Iqbal MA, Akhtar M, Ulmer C, Al-Dayel F, Paterson MC. FISH analysis in chromophobe renal-cell carcinoma. Diagn Cytopathol. 2000;22(1):3-6.
- Wilhelm M, Veltman JA, Olshen AB, Jain AN, Moore DH, Presti JC Jr, Kovacs G, Waldman FM. Array-based comparative genomic hybridization for the differential diagnosis of renal cell cancer. Cancer Res. 2002;62(4):957-60.
- Junker K, Weirich G, Amin MB, Moravek P, Hindermann W, Schubert J. Genetic subtyping of renal cell carcinoma by comparative genomic hybridization. Recent Results Cancer Res. 2003;162:169-75.